
Brand:
Model:
Producer:
Boron trichloride (BCl₃) is a highly reactive inorganic compound, widely utilized in chemical synthesis, semiconductor manufacturing, and materials science due to its strong Lewis acid properties and ability to form covalent bonds with various elements. Below is a concise summary of its core information:
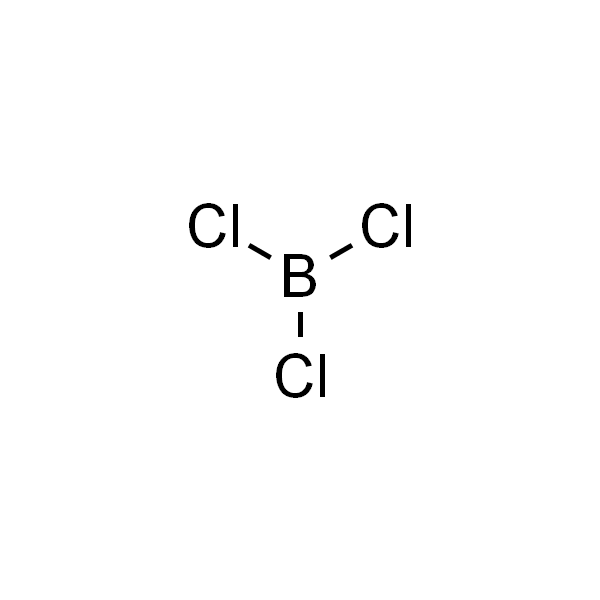
Chemical Name: Boron Trichloride
Molecular Formula: BCl₃
CAS Registry Number: 10294-34-5
Molecular Weight: 117.17 g/mol
Appearance & Odor: Colorless to pale yellow fuming liquid or gas (boils at 12.5°C), with a pungent, hydrochloric acid-like odor.
Boiling Point: 12.5°C (easily vaporizes at room temperature)
Melting Point: -107.3°C
Density: 1.349 g/cm³ (liquid, 0°C); 4.03 g/L (gas, 25°C)
Solubility: Reacts violently with water to form boric acid (H₃BO₃) and hydrochloric acid (HCl); soluble in non-polar organic solvents such as carbon tetrachloride, benzene, and chloroform.
Chemical Nature: A strong Lewis acid with an electron-deficient boron atom (sp² hybridized), enabling it to readily accept electron pairs and form complexes with amines, ethers, and other electron-donor compounds.
Primary boron source for chemical vapor deposition (CVD) and ion implantation processes: Used to deposit boron-doped silicon films, boron nitride (BN) coatings, and other semiconductor materials. Boron doping modulates the electrical conductivity of silicon wafers, critical for manufacturing transistors, diodes, and integrated circuits.
Serves as a catalyst or reagent in Friedel-Crafts reactions, polymerization of olefins, and synthesis of organic boron compounds (e.g., boronic acids).
Used to prepare high-purity boron, boron carbide (B₄C), and other boron-containing ceramics via reduction or pyrolysis.
Employed as a flux in metallurgy to remove oxide impurities from metal surfaces; utilized in the production of specialty glasses and optical fibers to adjust boron content and improve material properties.
Corrosion: Skin Corrosion/Irritation (Category 1A, H314) – Causes severe skin burns and eye damage.
Acute Toxicity: Inhalation Toxicity (Category 2, H331) – Toxic if inhaled.
Chemical Reactivity: Water-Reactive Substances (Category 1, H270) – Reacts violently with water, releasing flammable or toxic gases (HCl fumes).
Hazard Pictograms: GHS05 (Corrosion), GHS06 (Toxic)
Signal Word: Danger
Violent hydrolysis: Contact with water (including moisture in air) generates dense, corrosive HCl fumes, which can cause severe respiratory irritation and chemical burns.
Toxic inhalation: Vapors irritate the mucous membranes of the eyes, nose, and throat; high concentrations can lead to pulmonary edema, dizziness, and even unconsciousness.
Reactivity with bases: Reacts explosively with strong bases (e.g., NaOH) and reducing agents (e.g., metals).
Store in a cool, dry, well-ventilated, and corrosion-resistant storage cabinet, away from water, moisture, bases, and oxidizing agents.
Use gas cylinders with dedicated pressure regulators and leak-detection equipment; avoid physical impact or high temperatures.
Wear full personal protective equipment (PPE): chemical-resistant gloves, face shield, acid-resistant apron, and self-contained breathing apparatus (SCBA) when handling.
Inhalation: Immediately move the victim to fresh air; administer oxygen if breathing is difficult and seek medical attention promptly.
Skin/Eye Contact: Rinse thoroughly with copious amounts of water for at least 15 minutes; do not rub eyes and seek emergency medical help.
Spill: Isolate the contaminated area; use dry sand or inert absorbents to contain the spill (do not use water); dispose of waste via professional hazardous waste management companies.
BCl₃ must be handled under strict anhydrous conditions in industrial and laboratory settings. Its strong reactivity and toxicity require rigorous adherence to safety protocols to prevent accidents. High-purity grades (≥99.99%) are essential for semiconductor applications to avoid contaminating electronic components.